Preparation and formation mechanism of the highly
发布日期:2014-04-28 14:42 文章作者:焦翠燕 访问量:? 字体 :[ 大 ][ 中 ][ 小 ]
Cuiyan Jiao1, a*, Tiebing Xu1,b and Gang Ren1,c
1Environmental Science Research Institute of Hebei Province, No.30 Yaqing Street Shijiazhuang City, China
Keywords: Highly dispersive silver particles, Ascorbic acid, Growth mechanism, Self-dispersive function.
Abstract.The preparation of highly dispersive silver particles of average particle size of 1~2μm used for the front paste of the solar cell were carried out by rapid addition of ascorbic acid solution into the aqueous AgNO3 solution at room temperature. The dispersive quasi-spherical silver particles were obtained with the AgNO3 concentration of 0.1M, AgNO3/Ascorbic acid mass ratio of 1:0.8 without controlling the pH value of the system. Elevating the concentrations of the AgNO3 solution resulted in the formation of flowerlike structures. The formation mechanism was investigated,it was concluded that the growth process of the highly dispersive silver particles include three stages, first, the formation of the precursor, followed by the nuclei burst and the diffusive growth process due to formation of H+ ions adsorbed on the negative charged Ag nuclei as well as the steric effect of the ascorbic acid molecule, finally, when the pH value decreased dramatically to approaching the isoelectric point, the adsorbed H+ was released and the resulting primary particles in turn aggregated to form secondary particles.
Introduction
Up to now, the importance of well-defined silver particles, ranging from several nanometers to several micrometers, has been recognized in numerous applications[1]. Thus, many efforts have been made in the preparation of different morphologies and sizes of the uniform silver particles [2-8]. Among these, the precipitation of silver powder from homogeneous solutions is the most convenient method. Nano and micro-sized silver particles prepared by this method have been intensively studied not only due to their fundamental scientific interest but also because of their many useful applications in the catalysts, medical diagnostics, optical areas as well as electronic pastes[9]. Therefore, highly dispersive silver particles with narrow size distribution has attracted a great deal of attention in recent years[10-14].
It is well known that the agglomeration is a phenomenon normally occurred during the synthesis process, which had a great effect on the property and application of the silver particles. Therefore, how to prepare the silver particles with good dispersibility is an essential issue confronting the scientific researchers. Generally, the preparation of highly dispersive silver particles is conducted with the addition of the dispersant to hinder the aggregation of the particles[11]. In fact, the addition of dispersant in industry resulted in high chemicals consumption during the reaction, tedious washing process, abundant waste water production and even low efficiency to resolve the aggregation problem. Therefore, it is necessary to find an alternative route to prepare dispersive silver powder. Meanwhile, abundant literatures have reported on the dispersive mechanism of the dispersants such as PVP[15], gelatin[16] and PVA[11], which is generally due to the steric effect. At the same time, many researchers have employed the ascorbic aid used as the reductant for the preparation of silver powder [10-13, 17-20] due to its moderate reduction ability and the property of environmental friendly. However, the self-dispersive function of the ascorbic acid was rarely discussed up to now. Besides, the mechanism of crystal growth for the complex morphologies was hardly understood[18]. So, this paper reports the formation of quasi-spherical and flower-like forms of silver particles by reduction of silver ions with ascorbic acid. The growth mechanism of the particles was also proposed by investigating the zeta potential of the silver particles and the variation of the pH values during the reaction process. The formation process of the complicated architectures is discussed from view points of structural characterization and the influence of the experimental conditions.
Experiment
Silver nitrate and ascorbic acid of analytical grade(AR)were used as the starting materials for the preparation of silver powders. Deionized water with resistance of 16 MΩ·cm was used in all the cases. The dilute nitricacid and sodium hydroxide solution were used to adjust the pH values of the solution.
Silver particles were synthesized by the simple wet-chemical method. Typically, 50mL of 0.1M AgNO3 was added into a 250mL beaker and magnetically stirred at the rate of 140 rpm at room temperature. Then, the same volume of ascorbic acid solution with the same concentration was injected into the solution quickly. After 5 minutes, the silver particles could be separated easily from the solution by centrifugation and washed by pure water and ethyl alcohol three times respectively. The obtained powders were dried in the vacuum oven at 50℃ for 3h. The experimental conditions and the ending pH values of the solution (pHe) were presented in Table 1.
Table 1 The experimental conditions without controlling the pH value of the system.
The morphology of the product was observed by scanning electron microscopy (JEM-6360LV, JEOL). The mean particle size of the silver particles and the standard deviation of particle population were determined from image analyses of the micrographs of these particles, while data were analyzed by means of the Origin 8.0 software. X-ray diffraction analysis of the powder was carried out by an X-ray diffractometer (TTRⅢ, Rigaku) using CuKa radiation 40kV and 250mA at room temperature in air. Electrokinetic measurements of silver particles as a function of the pH in 1×10-3 mol·dm-3 aqueous solutions were evaluated with a Nano-ZS (MAL) instrument at 25℃.
Effect of the silver nitrate concentration on the morphologies of the silver particles
To address the influence of AgNO3 concentration on the morphology of the particles, the typical SEM images of the resulting products with different AgNO3 content are shown in Fig. 1a(0.1M),Fig. 1c(0.3M) and Fig. 1e(1.5M). The insets in Fig.1 (a,c,e) are the close-up view of the corresponding images respectively and Fig.1 (b, d, f) shows the corresponding particle size distribution histograms of silver micrometer-sized silver particles evaluated from the corresponding electron micrographs, in which the solid line is Gaussian fits of the data. It is suggested that the surfaces of the particles are not smooth and each particle showed in Fig.1 (a, c, e) is composed of submicrosized structures. It is clear that the particles are also well-separated spheres in macroscopy with a relatively narrow size distribution. The mean diameters of sample with 0.1M, 0.3M and 1.5M AgNO3 are 1.468μm, 2.619μm and 6.305μm, respectively, showing relatively high dispersibility, i.e., the diameters of the secondary particles as well as the sizes of the primary particles increase with the elevation of the AgNO3 concentration.
Meanwhile, the XRD patterns of the samples with different AgNO3 concentrations are shown in Fig. 2. The reflection peaks located at 38.1°, 44.3°, 64.4°, 77.5° and 81.5°are assigned to the (111), (200), (220), (311), and (222) crystal planes of Ag metal, respectively. They are well consistent with the JCPDS file (No. 65-2871), indicating the formation of Ag metal. The broadening of the peaks in the sample obtained by 0.1M and 0.3M AgNO3 indicated the formation of nano-sized particles[21]which is in consist with the corresponding SEM images, while the diffraction peaks in the sample obtained by 1.5M AgNO3 are relatively narrowed which indicated the formation of silver particless with high crystalline, deducing that the small petals composed of the flower-like structure is well crystallized. In addition, it is noticed that the ratio between the intensities of (111) and (200) diffraction peaks is much higher than the conventional value, which indicates that the particles are (111) oriented [6, 21].
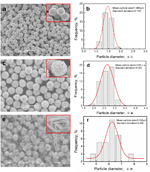 Fig.1 SEM image of the silver particles prepared with thedifferent concentrations of AgNO3 solution (a=0.1M, c=0.3M, e=1.5M, b, d, f were the particle size distribution histograms of silver micrometer-sized silver particles evaluated from the corresponding electron micrographs, in which the solid line is Gaussian fits of the data. The insets were the close-up view of the corresponding images )
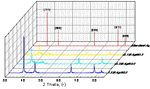 Fig.2 XRD patterns of the silver particles prepared with the different AgNO3 concentrations
Effect of pH variation on the morphology of the silver particles
The variation of the pH value was real-time measured and presented in Fig.3. It is presented that the pH value of the solution decreases dramatically at the beginning of the reaction and then almost steady thereafter. It is concluded that the nuclei and growth process for the rapid addition of ascorbic acid to the AgNO3 solution under intense agitation is very fast, which is in accordance with the kinetic model proposed by Egon Matijevic 22].
The zeta potential on the surface of the obtained silver particles is detected by Nano-ZS (MAL). The 10-3 mol·dm-3 KCl solution was prepared and different solution pH values at the range of 0~9 were adjusted by diluted HNO3 or NaOH solution. The surface Zeta potential of the micrometer-sized silver particles in 10-3 mol dm-3 KCl solution as a function of pH value is presented in Fig.3. It is found that the surface of the silver particles is generally negatively charged at the investigated pH range, and the zeta potential firstly increases and then decreases, finally keeps at a certain level with the increase of the pH value. It is suggested that the point of zero charge (PZC) of the obtained silver particle is approximately at the pH value of 1.0.
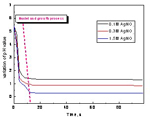 Fig.3 The variation of the pH value of the system as a function of time without adjustion
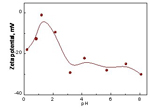 Fig.4 Zeta potential of the micrometer-sized silver particles in 10-3 mol dm-3 KCl solution as a function of pH
The possible formation mechanism of the high dispersive silver particles
Based on the above results, a growth mechanism for the formation of dispersive Ag particles is proposed. The formation of the dispersive silver particles with narrow size distribution is inferred to accomplish in three stages: first, the rapid feeding created the high supersaturated condition, the aqueous solution becomes black due to the formation of the intermediate product silver ascorbate[18], Second, the electron transfer in the silver ascorbate produces the nano-size nuclei, which grow by diffusive mass transfer due to the electrostatic barrier caused by the adsorption of H+ on the silver nuclei and the steric barrier from the cyclic structure of the ascorbic acid. The self-dispersive function of the ascorbic acid was illustrated as follows:
(1)
The molecules of the ascorbic acid are in the dynamic balance of heterogeneous types as ketone and enolate as shown in Eq.(1). Meanwhile, the enolate form is more stable than the ketone one. Besides, it is well known that Ag+ has two empty sp hybrid orbitals, which can accept lone pairs of hydroxyl O atom of the ascorbic acid to form coordination bond. The existence of a structure similar to large π bond in the ascorbic acid provided a high electron density, thus, it is nucleophilic and provides the effective electrophilic position for the reduction of Ag+. In addition, the steric effect caused by the cyclic structure of the ascorbic acid can prevent the aggregation of the powders as presented in Eq.(2). Therefore, the ascorbic acid plays the dual role of reductant and dispersant in the whole reaction process.
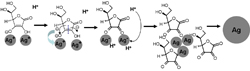 (2)
At last, when the pH value decreases dramatically to approximately 1 (as shown in Fig.4), which causing the surface potential of the nuclei approaching the isoelectric point, resulting in reduction of electrostatic barriers, thus promoting fast irreversible particle aggregation to form the secondary particles. The secondary particles are of narrow size distribution, which is clearly a diffusion-controlled process[23].
Conclusions
(1) The uniform, dispersive silver particles used for the front paste of the solar cell were prepared by a facile wet-chemical route.
(2) The ascorbic acid played a dual role of reductant and dispersant in the reduction process.
(3) The morphologies of the silver particles could be widely varied by varying AgNO3 concentrations. Higher AgNO3 concentration resulted in the flowerlike structures.
(4) The growth mechanism of the dispersive silver particles was illustrated as three stages, first, the formation of the intermediate product silver ascorbate as the precursor, second, the primary particles are formed by nuclei and diffusive growth process due to electrostatic barrier caused by the adsorption of H+ ions on the surface of the nuclei and the steric effect of the ascorbic acid. At last, with the approach of PZC, the electrostatic barrier was reduced, and the primary particles aggregated into secondary particles. With the adjustion of the pH value at the second stage, the adsorbed H+ ions on the silver nuclei was eliminated which favors for the aggregation and make the structure of the secondary particles more compact.
References
|
发布者:办公室
--摘自null
扫一扫在手机打开当前页
 冀公网安备 13010802001298号
冀公网安备 13010802001298号